Journal of Animal Research: v.10 n.2, p. 199-204. April 2020
DOI: 10.30954/2277-940X.02.2020.6
Novel Granulocytic Colony Stimulating Factor based Therapy for Morbidity Reduction in Pancytopenic Dogs with Babesia gibsoni
ABSTRACT
Vector borne pancytopenia is emerging as a life threatening entity in animals. In India babesiosis is one among the most prevalent tick-borne parasitic diseases of dogs caused by either Babesia gibsoni or Babesia canis. Bleeding due to thrombocytopenia and the concurrent anaemia and leukopenia were difficult to manage. This study assessed the efficacy of Filgrastim in pancytopenia associated with Babesia gibsoni in dogs presented to the Small Animal Medicine Referral Clinic, Madras Veterinary College. The therapeutic practices included Injection Filgrastim @ 10 µg/kg, SC, SID in combination with the standard triple therapy to manage the pancytopenia and the infection. Twenty numbers of PCR positive Babesia gibsoni dogs were used for this study. The animals were divided in to two groups based on therapeutic practices. First group consisted of dogs treated with triple therapy and the second group consisted of dogs treated and evaluated with Filgrastim along with triple therapy. The study showed that there was a significant increase in leukocyte count in Filgrastim treated group when compared to the other group. Integration of G-CSF along with standard triple therapy helped in better survival in pancytopenic dogs with Babesia gibsoni.
Keywords: Filgrastim, leukocyte, Babesia gibsoni, dog
Vector borne diseases are major challenges in animal health and productivity. Babesiosis is one among the most prevalent tick-borne parasitic diseases of dogs caused by either Babesia gibsoni or Babesia canis (Irwin, 2009; Solano-Gallego and Baneth, 2011). Canine babesiosis exhibits a wide range of presentations ranging from subclinical disease to serious illness characterized by fever, depression/lethargy, pale mucosae, anemia, thrompocytopenia, severe leukopenia, jaundice, splenomegaly, weakness and collapse associated with intra- and extra-vascular hemolysis. (Leisewitz et al., 2001; Jacobson, 2006; Irwin, 2009; Eichenberger et al., 2016). Bleeding due to thrombocytopenia associated with concurrent anaemia and leukopenia were difficult to manage. The aim of this study was to assess the efficacy of Filgrastim (G-CSF) in pancytopenic dogs with Babesia gibsoni and its effective therapeutic management.
How to cite this article: Pothiappan, P., Nagarajan, B., Selvaraj1, P., Vairamuthu, S. and Tirumurugaan, K.G. (2020). Novel granulocytic colony stimulating factor based therapy for morbidity reduction in pancytopenic dogs with Babesia gibsoni. J. Anim. Res., 10(2): 199-204.
MATERIALS AND METHODS
The present study was carried out using the medical records of the clinical cases presented to the Small Animal Medicine Referral Clinic and Emergency Critical Care Medicine Referral Clinic of Madras Veterinary College, Chennai. During the period 2018-19, a total of 120 cases with babesiosis were attended and their medical records were screened. 54 out of their 120 were affected with pancytopenias. Only 20 dogs irrespective of their breed, age and sex fulfilled our criteria were selected for this study. All these twenty dogs were PCR confirmed for Babesia gibsoni. The animals were divided in to two groups based on therapeutic practices. First group consisted of dogs treated with triple therapy and the second group consisted of dogs treated with Filgrastim along with triple therapy. The records of apparently healthy dogs brought for routine general health check up were taken as control group. Comparative assessments of haematobiochemical evaluations before and after treatment were compared with various variables on 0 day, 7th day and 28th days.
Table 1: Design of the therapeutic regimen group
Parameters studied
Haematology
The haematological parameters were estimated as per standard protocols described by Harrus et al. (1999). These haematological studies were done using automated haematology analyzer® (Mindray-BC-2800 VET, Shenzhen Mindray Bio-Medical Electronics Co., Ltd., China).
Biochemical parameters
About 2.0 ml of blood was collected in a vacutainer without anticoagulant for biochemical studies. Serum was separated by centrifugation at 1500 rpm for 20 minutes testing was run in a semi automated biochemical analyser (A-15 Biosystem random access analyzer®, Biosystem, Barcelona, Spain). Manufacturer recommended biochemical kits (Agape Diagnostics, India) were used for quantitative estimations of BUN, Creatinine, Total protein, Albumin, Alkaline phosphatase, Alanine aminotransferase, Total and direct Bilirubin, Calcium and Phosphorous. These biochemical estimations were carried out as per the standard protocols detailed by Whitbread (2010).
Polymerase chain reaction (PCR) for diagnostic comparisons
Blood samples were used for PCR testing. DNA isolation kit (Quick-DNATM Miniprep Kit®, Zymo Research) was used for the parasite DNA extraction. Genomic DNA was isolated from the whole blood of infection free dogs which were screened as per Wardrop et al. (2016) and the same was used as negative control along nuclease free water. Singular PCR was performed for the detection of parasite DNA.
Table 2: Details of the primers used in singular PCR
RESULTS AND DISCUSSION
Pancytopenic dogs are the emerging clinical challenge for Veterinary Hospitals throughout India and elsewhere. Vector borne diseases remain day today challenge as existing therapies are inadequate to manage such animals. Novel therapies are used to arrest the decline in cell populations and to save the animals from death. This study helped to assess the efficacy of emerging therapies. In this study the haematological parameters of group I and II dogs on 0 day, 7th day and 28th day were comparatively assessed (Table 3 and 4). Dogs infected with B. gibsoni showed marked reduction in all the parameters except that of monocytes when compared to the normal dogs. After treatment there was a significant increase in the value of Haemoglobin, PCV and platelet values (day 7 and 28) when compared to the pre treatment values (Day 0). The numbers of RBCs and WBCs had not increased significantly on 7th day post treatment when compared to pre treatment values (Day 0); however the values were significantly higher between day 7 and 28 in the Group I which underwent standard triple therapy. The numbers of WBCs were significantly higher post treatment (day 7) when compared to pre treatment values (Day 0) but the values were not significantly higher between day 7 and 28 in Group II which underwent G-CSF administrations along with standard therapy.
Biochemical parameters of group I and II dogs on 0 day, 7th day and 28th day were presented in (Table 5 and 6). Dogs infected with B. gibsoni showed marked increase in the studied all the parameters except that of Creatinine, Total protein, Albumin, Calcium and Phosphorous when compared to normal values. In group I, there was a significant decrease in the ALP values post treatment (day 7 and 28) when compared to pre treatment values (Day 0) but other parameters were though altered statistically not significant. In group II, there was a significant increase in the Total protein values post treatment (day 7 and 28) when compared to pre treatment values (Day 0) but other parameters were though altered statistically not significant.
Table 3: Haematological parameters of Group I animals
Table 4: Haematological parameters of Group II animals
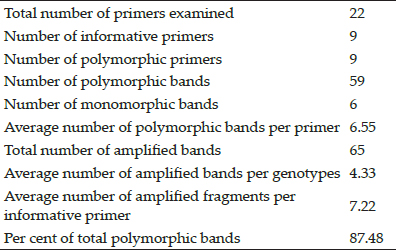
*P<0.05, **P<0.01, NS: Non significant. Mean bearing the same superscript didn’t differ significantly between the days otherwise different significantly @ 1% level of significant.
Table 5: Biochemical parameters of Group I animals
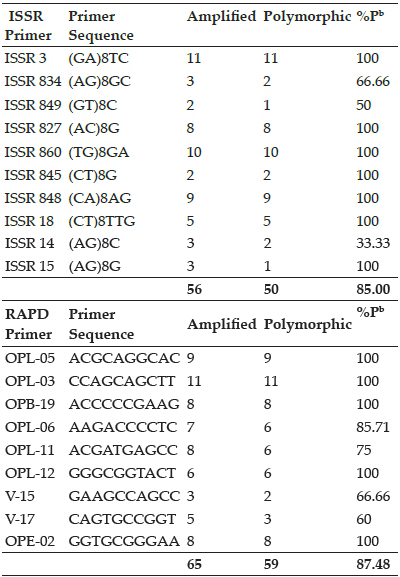
Table 6: Biochemical parameters of Group II animals
*P<0.05, **P<0.01, NS: Non significant. Mean bearing the same superscript didn’t differ significantly between the days otherwise different significantly @ 1% level of significant.
In the present study, the common haematobiochemical findings observed were anemia, thrombocytopenia, leukopenia, hypoproteinemia, hypoalbuminemia increases in the serum activity of BUN, Creatinine, ALT, ALP and hyperbilirubinemia. These findings correlated with the earlier studies reported by Bourdeau and Guelfi (1995), Vercammen et al. (1997) and Lobetti (2000). Elevated in the activity of aspartate aminotransferase (AST) and alanine amino transferase (ALT), hyperbilirubinemia, hypoalbuminemia, and electrolyte and acid-base abnormalities were reported to be common findings in Babesia affected dogs (Lobetti, 2011; Zygner et al., 2012; Ruiz de Gopegui et al., 2007). The same was observed in this study too.
In canine babesiosis is characterised by both anemia and thrombocytopenia. The anemia is initially mild, normocytic and normochromic, then turns in to macrocytic, hypochromic, and regenerative as the disease progresses. Reticulocytosis occurred proportional to the severity of the anaemia. Moderate to severe thrombocytopenia is the most common finding in canine babesiosis, independent of the subspecies of parasite involved (Lobetti, 2001). Leukocyte abnormalities are inconsistently observed and there may includes leucocytosis, leucopenia, neutrophilia or neutropenia, lymphocytosis, and eosinophilia (Vercammen et al., 1997). Thrombocytopenia with no obvious hemorrhage during the clinical examination, platelet destruction, increase platelet sequestration, and decreased platelet production, were linked to thrombocytopenia (Feldman et al., 1988). Similar findings were observed in this study too.
Though clinical signs are suggestive of diagnosis, its confirmation needs PCR testing. PCR based assays offered greater sensitivity and specificity in the detection of parasite DNA in blood samples detailed by Kledmanee et al. (2009). In this study the PCR helped the confirmation of the diagnosis.
Suzuki et al. (2007) and Lin and Huang (2010) reported of success with the treatment of B. gibsoni using triple antibiotic combinations: Doxycycline (5 mg/kg, orally, twice daily), Clindamycin (25 mg/kg, orally, twice daily), Metronidazole (15 mg/kg, orally, twice daily). Besides these, many other drug combinations were reported to be effective for babesiosis in dogs (Vial and Gorenflot, 2006; Irwin, 2009; Solano-Gallego and Baneth, 2011; Beugnet and Moreau, 2015). However not all of them effectively addressed the pancytopenic complications in affected dogs.
Current novel agents are used in managing pancytopenia. Filgrastim is a granulocyte colony-stimulating factor (G-CSF) used it induces a dose-dependent production of granulocyte progenitor cells within the bone marrow. The therapeutic regimen included high doses of Filgrastim in combination with other standard therapies to manage the pancytopenia and infection (Palacios et al., 2017). Filgrastim is a recombinant Methionyl Human Granulocyte Colony Stimulating Factor (r-metHuG-CSF) analog and is being used to stimulate the proliferation and differentiation of granulocytes in humans undergoing chemotherapy with granulocyte values below 0.5 G/l and who are likely to be threatened by infections, including sepsis even possibly with a fatal outcome (Novotny et al., 1995). The administration of exogenous G-CSF induces a dose-dependent production of granulocyte progenitor cells within the bone marrow. (Johnston and Battersby, 2012; Lane et al., 2012).
Nonregenerative pancytopenia in dogs are commonly associated with monocytic ehrlichiosis and such dogs had poor prognostic signs like neutropenia, thrombocytopenia, and severe anemia. In these dogs were treated with antibiotics and a short course of high-dose Filgrastim (50 µg/kg, SC, q 48h for 4 days) to stimulate bone marrow response. In addition prednisone was given to decrease peripheral platelet destruction. Also iron supplements were given to compensate for the iron deficiency in the bone deposits. By the second week after treatment with Filgrastim, there was a reduction in the number of petechiae, epistaxes, and gingival bleeding episodes, Palacios et al. (2017). In this study also the Filgrastim integrated triple therapy effectively managed the pancytopenia in affected dogs.
These studies had proved that Filgrastim was found to be very effective and successful in managing dogs with leukopenia. Filgrastim administration resulted in increase in the peripheral blood leukocyte counts after single administration. Haematobiochemical evaluations before and after treatment were compared and they showed a significant increase in leukocyte count in Filgrastim treated group when compared to the other group. Filgrastim was found to be very effective clinical therapy in managing the pancytopenic dogs. Though there was an increase in cell count after triple therapy, it was not statistically significant. In contrast, the G-CSF treated dog had shown significantly increased cell count and their by helped better recovery. Out of the ten dogs treated with triple therapy only three had died; such mortality was not observed in G-CSF treated dogs. Hence the efficacy of G-CSF in morbidity reduction is clearly evident.
CONCLUSION
Integration of G-CSF along with standard triple therapy helped in better survival of Babesia affected dogs. Injection Filgrastim helped to increase the count of leukocytes within 24 hours of administration resulted in a very effective and successful clinical response in dogs complicated with pancytopenia. This G-CSF treatment plan can substantially change the clinical course of the disease for better and decrease morbidity when compared to conventional treatments employed in pancytopenic dogs.
REFERENCES
Beugnet, F. and Moreau, Y. 2015. Babesiosis. Rev. Sci. Tech., 34: 627–639.
Bourdeau, P. and Guelfi, J.F. 1995. La babésiose canine à Babesia canis, Point Vet., 27: 11–24.
Eichenberger, R.M., Riond, B., Willi, B., Hofmann-Lehmann, R. and Deplazes, P. 2016. Prognostic markers in acute Babesia canis infections. J. Vet. Intern. Med., 30: 174–182.
Feldman, B.F., Thomason, K.J. and Jain, N.C. 1988. Quantitative platelet disorders. Vet. Clin. N. Am., 8(1): 35–49.
Foldvari, G., Hell, E. and Farkas, R. 2005. Babesia canis canis in dogs from Hungary: detection by PCR and sequencing. Vet. Parasitol., 127: 221–226.
Harrus, S., Waner, T., Bark, H., Jongejan, F. and Cornelissen, A.W. 1999. Recent advances in determining the pathogenesis of canine monocytic ehrlichiosis. J. Clin. Microbiol., 37: 2745–2749
Irwin, P.J. 2009. Canine babesiosis from molecular taxonomy to control. Parasit. Vectors., 2(1): 4-5.
Jacobson, L.S. 2006. The South African form of severe and complicated canine babesiosis: clinical advances 1994–2004. Vet. Parasitol., 138: 126–139.
Johnston, M. and Batters, I. 2012. Control of off-label use of medicines. Vet. Rec., 171: 51.
Kledmanee, K., Suwanpakdee, S., Krajangwong, S. and Chatsiriwech, J. 2009. Development of multiplex polymerase chain reaction for detection of Ehrlichia canis, Babesia spp and hepatozoon canis in canine blood. Se. Asian J. Trop. Med., 40(1): 35-9.
Lane, A.E., Chan, M.J. and Wyatt, K.M. 2012. Use of recombinant human granulocyte colonystimulating factor prior to autologous bone marrow transplantation in dogs with lymphoma. Am. J. Vet. Res., 73: 894–899.
Leisewitz, A.L., Jacobson, L.S., De Morais, H.S. and Reyers, F. 2001. The mixed acid-base disturbances of severe canine babesiosis. J. Vet. Intern. Med., 15: 445–452.
Lin, M.Y. and Huang, H.P. 2010. Use of doxycycline– enrofloxacin– metronidazole combination with and without injections of diminazene diaceturate to treat naturally occurring canine babesiosis caused by Babesia gibsoni. Acta. Vet. Scand., 52: 27-28.
Lobetti, R.G. 2000. Canine babesiosis. In: M.J. Day, A. Mackin and J.D. Littlewood, BSAVA Manual of Canine and Feline Haematology and Transfusion Medicine, BSAVA, Gloucester., 85–91.
Lobetti, R.G. and Jacobson, L.S. 2001. Renal involvement in dogs with babesiosis. J. S. Afr. Vet. Assoc., 72(1): 23–28.
Lobetti, R. 2011. Changes in the serum urea: creatinine ratio in dogs with babesiosis, haemolytic anaemia, and experimental haemoglobinaemia. Vet. J., 191: 253–256.
Novotny, J., Zvarova, M., Prazakova, L., Jandlova, M. and Konvickova. L. 1995. GCSF in the treatment of patients with chronic aplastic anemia with severe neutropenia, Vnitr. Lek., 41(10): 692-5.
Palacios, M., Arteaga, R. and Gustavo, C. 2017. High-dose filgrastim treatment of nonregenerative pancytopenia associated with chronic canine ehrlichiosis. Topics. In. Compan. An. Med., 32: 28–30.
Ruiz de Gopegui, R., Penalba, B. and Goicoa, A. 2007. Clinicopathological findings and coagulation disorders in 45 cases of canine babesiosis in Spain. Vet. J., 174: 129–132.
Solano Gallego, L. and Baneth, G. 2011. Babesiosis in dogs and cats – expanding parasitological and clinical spectra. Vet. Parasitol, 181: 48–60.
Suzuki, K., Wakabayashi, H., Takahashi, M., Fukushima, K., Yabuki, A. and Endo, Y.A. 2007. Possible treatment strategy and clinical factors to estimate the treatment response in Bebesia gibsoni infection. J. Vet. Med. Sci., 69(5): 563-8.
Vercammen, F., De Deken, R. and Maes, L. 1997. Haematological and biochemical profile in experimental canine ‘babesiosis’ (Babesia canis), Vlaams Diergences- kundig Tijdschrift., 66: 174–178.
Vial, H.J. and Gorenflot, A. 2006. Chemotherapy against babesiosis. Vet. Parasitol., 138: 147–160.
Wardrop, K.J., Birkenheuer, A., Blais, M.C., Callan, M.B., Kohn, B., Lappin, M.R. and Sykes, J. 2016. Update on Canine and Feline Blood Donor Screening for Blood-Borne Pathogens. J. Vet. Intern. Med., 30: 15–35.
Whitbread, T.J. 2010. Diagnostic procedures for the private practice laboratory. In merck’s vet manual, 8th ed., Merck & Co., Inc., Kenilworth, NJ, USA: 201-221.
Zygner, W., Gojska Zygner, O. and Norbury, L.J. 2012. Increased AST/ALT ratio in azotaemic dogs infected with Babesia canis. Pol. J. Vet. Sci., 15: 483–486.